ClearPoint Neuro Announces EU MDR Certification for ClearPoint Navigation Software Version 3.0.2, Expanding Access to the Latest Operating Room Navigation Platform in Europe
SOLANA BEACH, CALIFORNIA / ACCESS Newswire / January 22, 2026 / ClearPoint Neuro, Inc. (NASDAQ:CLPT) (the "Company"), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today announced it has received EU MDR Certification for its ClearPoint Navigation Software Version 3.0.2.
"By achieving CE Mark for the ClearPoint Navigation 3.0.2 software, we are able to unify our global navigation platform which we believe will enable consistent training and hospital IT support," commented Mazin Sabra, Chief Operating Officer at ClearPoint Neuro. "We expect that this will also help us to not only satisfy our biopharma partners who want a global solution, but also reduce our operating costs and drive economies of scale."
ClearPoint Navigation Software Version 3.x was first introduced in the United States following FDA clearance 11 months ago and has already been adopted by most US customers. "By releasing our latest version of software to our EU customers, we are excited to offer feature refinements developed through years of valuable user experience and feedback," stated Tim Orr, VP of Software Development at ClearPoint Neuro. "The 3.0.2 release represents an important milestone which will allow us to unify US and EU customers on the same navigation platform."
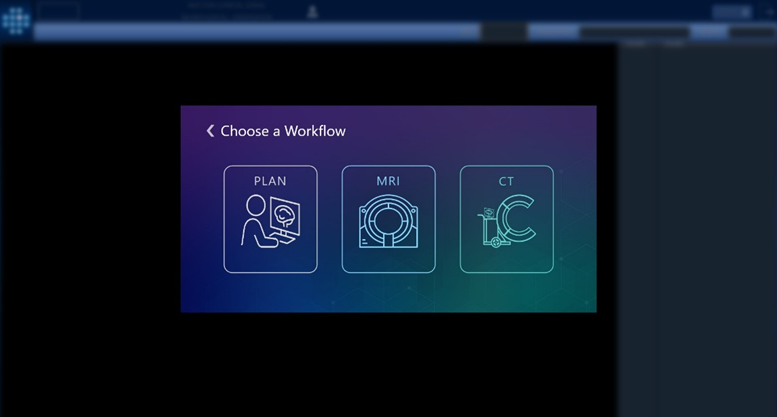
Version 3.0.2, now available in Europe, introduces an intraoperative CT workflow that builds on over a decade of experience in stereotactic procedures. While earlier generations of ClearPoint software supported MRI-guided workflows exclusively, the 3.0.2 release extends ClearPoint navigation capabilities to the operating room. By offering compatibility with both intraoperative CT and Cone-beam CT imaging, the software is designed to increase access to precision-guided neurosurgery for facilities without intraoperative MRI capabilities. The ClearPoint Navigation Software Version 3.0.2, when used in conjunction with the SmartFrame XG stereotactic frame, is intended to provide precise stereotactic guidance when placing instruments or devices during neurosurgical procedures. These procedures include biopsies, catheter and electrode insertion including deep brain stimulation (asleep or awake) lead placement.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as pre-clinical development services for controlled drug and device delivery. The Company's flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct CNS delivery of therapeutics in pre-clinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company's field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
This press release contains forward-looking statements within the context of the federal securities laws, including the Company's expectation for the future market of its products and services, expectations for reducing costs and gaining efficiencies by enablement of a unified software platform, and other performance and results. These forward-looking statements are based on management's current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: global and political instability; geopolitical trends, such as protectionism and economic nationalism; the introduction of or changes in tariffs, sanctions, or trade barriers; supply chain disruptions and macroeconomic and inflationary conditions; future revenue from sales of the Company's products and services; the Company's ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company's products and services in their delivery of therapies; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company's ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products. For a detailed description of the Company's risks and uncertainties, you are encouraged to review its documents filed with the SEC including the Company's recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Contact:
Media Contact:
[email protected]
Investor Relations:
Danilo D'Alessandro, Chief Financial Officer
(888) 287-9109
[email protected]
SOURCE: ClearPoint Neuro, Inc.
Information contained on this page is provided by an independent third-party content provider. XPRMedia and this Site make no warranties or representations in connection therewith. If you are affiliated with this page and would like it removed please contact [email protected]